Import, Distribution and Sale of Medical & Research Products
Import, Distribution and Sale of Medical & Research Products
We possess advanced expertise in importing and distributing medical and research products from Asia to the European market. When we collaborate with you as a manufacturer, we provide comprehensive support throughout the entire product development lifecycle. Our assistance encompasses various regulatory matters, import-related challenges, and certification issues. We specialize in tailoring unique solutions for you to enter the European market.
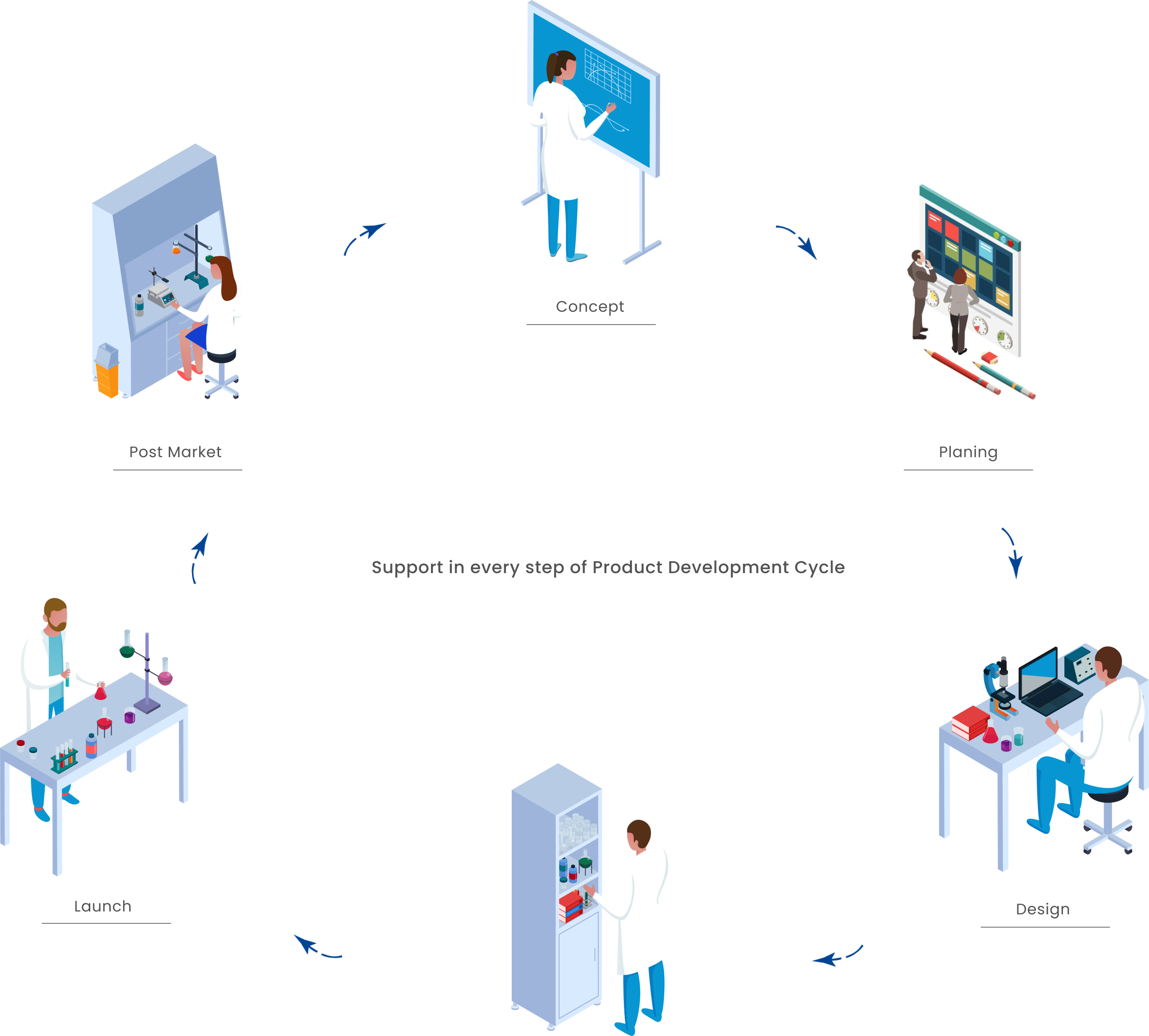
Concept:
We support you with consulting during the initial evaluation of the possible development of commercial products regarding laws, regulations, norms and needs in the European market.
Planning:
Design:
We offer assistance throughout the product design and manufacturing process, including verification or validation.
Validation:
The knowledge gained from analytical performance studies or (valid) scientific literature about aspects of clinical and analytical performance of the IVD and, if applicable, clinical performance studies are summarized in a performance evaluation report.
Launch:
We make sure that every distribution and marketing channel is optimally tuned for the launch of your product.
Post Marketing Surveillance:
We support you with consulting during the initial evaluation of the possible development of commercial products regarding laws, regulations, norms and needs in the European market.
Planning:
- Plan and Assistance for the Certification Process
- Performance Evaluation Plan (PEP)
Design:
We offer assistance throughout the product design and manufacturing process, including verification or validation.
Validation:
The knowledge gained from analytical performance studies or (valid) scientific literature about aspects of clinical and analytical performance of the IVD and, if applicable, clinical performance studies are summarized in a performance evaluation report.
Launch:
We make sure that every distribution and marketing channel is optimally tuned for the launch of your product.
Post Marketing Surveillance:
- Part B of the new IVDR Annex XIII deals with the post-market phase and describes the requirements for tracking post-market performance.
- PMPF (post-market performance follow-up) refers to the concept that you must continuously review the performance evaluation report to ensure that it reflects the current state of the art.
- We help you to design and implement the PMPF Plan successfully. This will benefit the product competitiveness immensely.
